Our Science
Recent Publications
Vaccination with Polyclonal Antibody Stimulator (PAS) Prevents Pancreatic Carcinogenesis in the KRAS Mouse Model
The incidence of pancreatic cancer is increasing signifi-cantly and will soon become the second leading cause of cancer-related deaths in the United States. We have previ-ously shown that the gastrointestinal peptide gastrin, which is only expressed in the fetal pancreas and not in the adult pancreas, is activated during pancreatic carcinogenesis where it stimulates growth in an autocrine fashion. Continue Reading
Gastrin Vaccine Alone and in Combination With an Immune Checkpoint Antibody Inhibits Growth and Metastases of Gastric Cancer
Gastric cancer is a leading cause of cancer-related deaths worldwide. Recently, clinical studies have demonstrated that many of those with advanced gastric cancer are responsive to immune checkpoint antibody therapy, although the median survival even with these new agents is less than 12 months for advanced disease. Continue Reading
Vaccine against gastrin, a polyclonal antibody stimulator, decreases pancreatic cancer metastases
A predominant feature of pancreatic cancer is its propensity to metastasize early and frequently (64). In fact, 90% of patients have advanced disease at the time of presentation (9). Although the retroperitoneal location and nonspecific symptoms may contribute to late stage diagnosis of pancreatic cancer, some characteristic features associated with this malignancy may facilitate metastases. One of the first steps in the metastatic cascade is invasion, a process that involves the loss of cell-cell adhesion and changes in the extracellular matrix to promote motility and migration (24). Continue Reading
Gastrin vaccine improves response to immune checkpoint antibody in murine pancreatic cancer by altering the tumor microenvironment
Pancreatic cancer has been termed a ‘recalcitrant cancer’ due to its relative resistance to chemotherapy and immunotherapy.
This resistance is thought to be due in part to the dense fibrotic tumor microenvironment and lack of tumor infiltrating
CD8 + T cells. The gastrointestinal peptide, gastrin, has been shown to stimulate growth of pancreatic cancer by both a paracrine
and autocrine mechanism. Interruption of gastrin at the CCK receptor may reduce tumor-associated fibrosis and alter
tumor immune cells. Polyclonal Ab Stimulator (PAS) is a vaccine that targets gastrin and has been shown to prolong survival
of patients with pancreatic cancer. Here, we report that PAS vaccination monotherapy elicits both a humoral and cellular
immune response when used in immune competent mice-bearing pancreatic tumors and that PAS monotherapy produced a
marked T-cell activation and influx of CD8 + lymphocytes into pancreatic tumors. Continue Reading
Our Target
Normal Function of Gastrin
- Gastrin is produced in the G-cells of the stomach.
- Gastrin acts on CCK-B receptors to produce the gastric acid needed for digestion.
- CCK-B receptors are normally located in the stomach, colon, and pancreas.
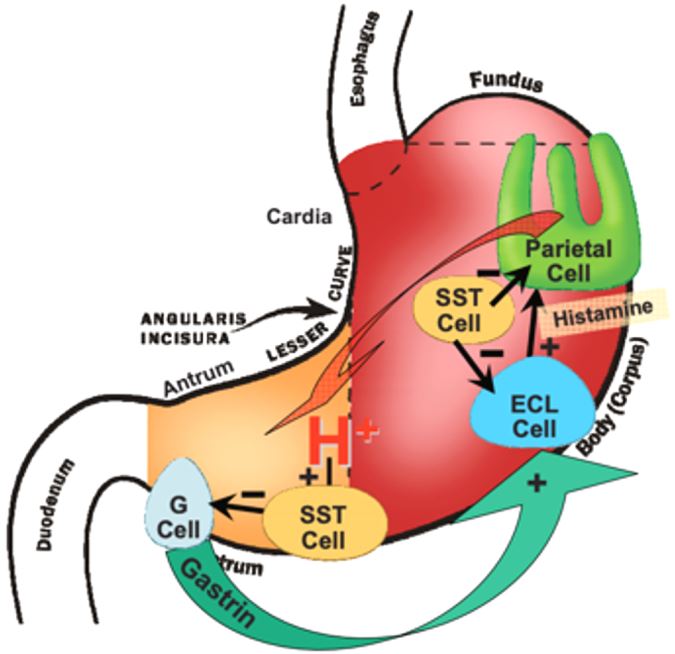
Stanislaw J. Konturek, Jagiellonian University Medical College, Cracow, Poland
http://www.jpp.krakow.pl/journal/archive/04_11/articles/15_article.html
CCK-B Receptors are Overexpressed in some Cancers
- Gastrin is also secreted by these cancers to create an autocrine loop.
- Gastrin stimulates growth of these cancers.
- PAS blocks the trophic effects of gastrin and disrupts tumor growth.
- Growth promoting effect of gastrin peptides suggests gastrin system has broad carcinogenic significance.*
* Burkitt et. al. World J Gastroenterol 2009 January 7; 15(1): 1-16. “There is now accumulating evidence that altered local and plasma concentrations of gastrin may play a role during the development of various gastric tumors.“
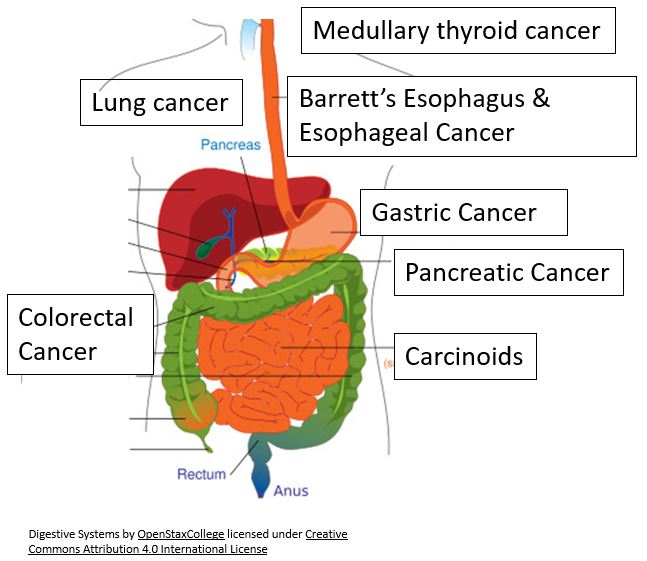
Gastrointestinal Cancers Present a Unique Challenge
- GI cancers as a group are the most common cancers in the U.S. (2019 prevalence below)
- Gastric ~100,000
- Pancreatic ~75,000
- Esophagus ~47,000
- Colorectal ~1.3 M
- Often diagnosed at an advanced stage
- Extremely poor prognosis
- Few effective treatment options for advanced patients
Polyclonal Antibody Stimulator (PAS) inhibits gastrin, minimizing cellular proliferation and tumorigenesis in GI cancers
Source: GI Alliance, seer.cancer.gov
Our Product
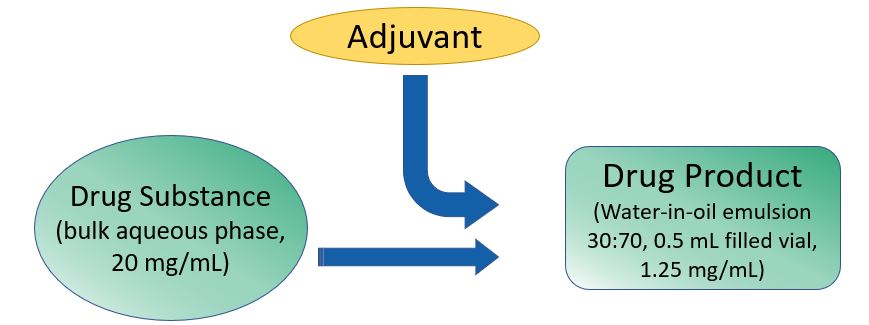
- PAS is a peptide-conjugate that includes an epitope of human gastrin (G17) linked to carrier diphtheria toxoid.
- PAS activates B-cells and elicits specific and high-affinity anti-gastrin Abs, which prevent trophic activity of gastrin at the CCK-B receptor.
- PAS produces a G17 antibody response in 50-80 % of subjects.
- PAS is administered in humans as an intramuscular injection over a multi-week regimen.
The PAS vaccine was discovered at the University of Nottingham and developed into Phase 3 by Aphton. It was acquired Cancer Advances in 2008.
GMP material has been manufactured for 22 clinical trials, including two Phase 3 trials. Robust, cost efficient production.
PAS has previously been called G17DT and Gastrimmune.
Excellent safety and tolerability profile.
Cancer Advances exclusively owns PAS and is funding and managing all aspects of the PAS gastrin vaccine program.
PAS Clinical Program Overview
22 clinical studies have been completed in over 1,500 subjects, including 2 placebo-controlled Phase 3 studies
PAS has been actively investigated in three different indications:
6 pancreatic cancer studies, Phases 1-3
-Total exposure: n = 6545
Gastric cancer studies, Phases 1-2
-Total exposure: n = 234
5 colorectal cancer studies, Phases 1-2
-Total exposure: n = 368
Excellent safety and tolerability profile. Injection site irritation only notable AE. No adverse reactions or autoimmune effects were detected effecting the normal functions of gastrin.
Provides survival benefit in pancreatic and metastatic gastric cancer patients that show an immune response (≥65% of all treated patients)
Recent pre-clinical work by Georgetown University validates efficacy as a monotherapy and supports further exploration of PAS in combination with checkpoint inhibitors
Pioneering new medicines and redefining possibilities for cancer patients.
Cancer Advances
Westpark Corporate Center
4364 South Alston Avenue,
Suite 105 Durham, North Carolina
USA 27713
